Q: What is the best solvent for pimonidazole HCl (Hypoxyprobe-1)?
A: Pimonidazole HCl is the hydrochloride salt of the weak base, pimonidazole, and is very soluble in aqueous solutions including neutral buffered saline (116 mg/mL or 400 millimolar). This permits the use of small volumes (0.1-0.5 mL) for intraperitoneal or intravenous injections of pimonidazole HCl in animal studies.
Q: What dose of pimonidazole HCl) should be used for hypoxia marking?
A: The extent of pimonidazole binding in
hypoxic tissue will depend on the rate of bioreductive activation and on tissue
exposure to pimonidazole (exposure = concentration x time). It is hard to
predict the rate of reductive metabolism but the effect of exposure can be
examined. In the following calculations, concentrations are given in terms of
pimonidazole hydrochloride (MW 290.7).
Strong hypoxia staining is observed in spheroids
exposed in vitro to 58mg/kg (concentration in medium) for 1 hour. Exposure =
58mg/kg x 1 hour = 58mg/kg-hour.
Strong staining for hypoxia in tumor tissue and
in normal epithelia is obtained in humans with a dose of 0.5gm/m2 (ca 14mg/kg)
where the plasma half-life of pimonidazole is 5 hours. Exposure = 14 mg/kg x 5
hours = 70 mg/kg-hour.
Strong staining for hypoxia in mouse tumor tissue
is obtained with 60 mg/kg where the plasma half-life of pimonidazole is 0.25
hours. Exposure = 60mg/kg x 0.25 hours = 15 mg/kg - hour.
In summary, for small animals of uniform size
such as laboratory rats and mice, a dose of pimonidazole HCl of 60 mg/kg body
weight is recommended as a good balance between effectiveness and economy.
Doses ranging from 30 mg/kg to 400 mg/kg have been used in mice and rats
without toxicity or altered oxygen levels due to blood flow effects with the
exception that blood flow effects have been observed at doses above 100 mg/kg
of pimonidazole for tumors implanted in the hind legs of mice. Caution must be
taken, therefore, when doses > 100 mg/kg are used in hind leg tumor models.
For larger animals with non-uniform body size,
the dose is typically calculated on the basis of surface area. For humans, the
recommended dose is 0.5 gm/m2 while for dogs a dose of 0.28 gm/m2 has been
used.
Q. Does Hypoxyprobe™-1 penetrate hypoxic brain and brain tumor tissue?
A: Although Hypoxyprobe™-1 is water soluble, its corresponding free base has an octanol water coefficient of 8.5 and, as a result, the marker freely penetrates into both brain and brain tumor tissue.
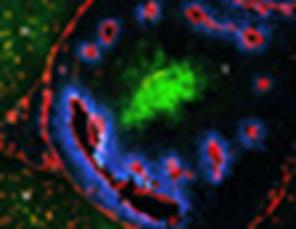
Immunofluorescence staining of a frozen section of a rat brain tumor. Vasculature: ME 9F1; Red. Hypoxia: Pimonidazole adducts; Green. Perfusion: Hoechst 33342; Blue. Normal brain (N) and tumor tissue (T). Original magnification was x 200. Note the regular pattern of vasculature in normal brain versus poorly organized vasculature in tumor tissue (Bernsen et al, Journal of Neurosurgery 93: 449-454, 2000; by permission).
Q. Is pimonidazole HCl the best probe for detecting hypoxia in vivo?
A: Pimonidazole HCl, the old standard
immmunohistochemical hypoxia marker, has real advantages including high water
solubility (116 mg/mL in saline) that allows administration as small volume
injections ip or iv. Markers such as the hexafluorinated CCI-103F have aqueous
solubility of 10 millimolar or less and are usually administered as ip emulsions
of peanut oil and DMSO in order to avoid hemodilution.
Solid pimonidazole HCl is very stable (years)
when stored at room temperature or at 4oC. Concentrated aqueous solutions of
pimonidazole HCl are very stable (years) when stored at 4oC in the absence of
light. Mouse and rabbit antibodies to pimonidazole adducts are stable for ≥ one
year stored at 4oC.
LD50(7days) for pimonidazole in mice is 728
mg/kg classifying it as a non-hazardous chemical of low toxicity.
Although pimonidazole HCl is very water soluble,
pimonidazole as a free base has a high octanol-water partition coefficient of
8.5 and it readily penetrates all tissues including brain. In fact, it
accumulates in most tissues 3 fold above plasma levels so that its effective
tissue concentration is much higher than other 2-nitromidazole hypoxia markers.
Pimonidazole binding can be detected by a wide
range of techniques that include: immunofluorescence in frozen fixed tissue
sections; immunoperoxidase staining in formalin fixed paraffin embedded tissue
sections; ELISA; or, flow cytometry.
Q. Can the monoclonal antibody to Hypoxyprobe™-1 adducts be used on mouse tissue?
A: Yes. For formalin fixed paraffin embedded tissues we recommend a peroxidase F(ab)2 secondary antibody strategy. This gives a very clean background and is applicable to a variety of animal species.
Q.What is the mechanism for the activation and binding of pimonidazole to hypoxic cells?
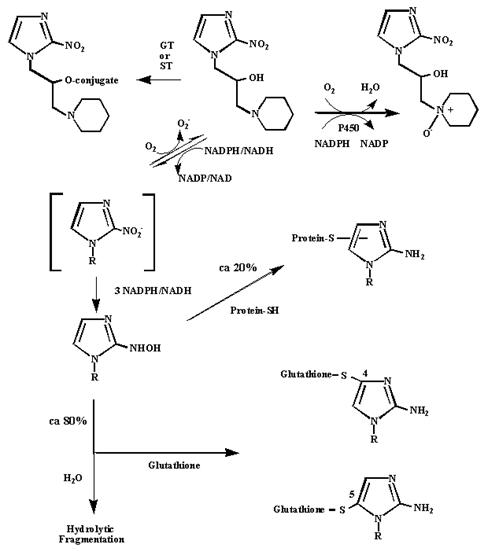
A: Varghese et al. showed that hypoxic cells bind 2-nitroimidazoles to peptide thiols such as glutathione. The current view of the metabolism of pimonidazole is summarized in the scheme below. O2 competes for the addition of the first electron to pimonidazole which accounts for the pO2 dependence of activation and binding. On the basis of test tube experiments, it is estimated that ca 20 % of activated pimonidazole binds to cellular thiols and ca 80% does not bind but is fragmented by reaction with water. Pimonidazole is subject to oxidative metabolism leading to easily excretable N-oxide, sulfate and glucuronate derivatives (ST = sulfotransferase; GT = glucuronly transferase). These oxidative pathways do not appear to interfere with the utility of pimonidazole as a hypoxia marker.
Q. What is the concentration of the IgG1 monoclonal antibody in the antibody solution supplied with the Hypoxyprobe kits?
A: The concentration of the mouse IgG1
monoclonal antibody in the exhausted hybridoma solution supplied in the
Hypoxyprobe-1 Kit is ca 60 micrograms/mL
The concentration of affinity purified IgG1 in the
FITC-conjugated MAb in the Hypoxyprobe-1 Plus Kit; the Hypoxprobe-1 Green Kit;
the Hypoxyprobe-1 Red549 Kit; the Hypoxyprobe-1 RedAPC Kit; and the
Hypoxyprobe-1 Biotin Kit is ca 500 micrograms/mL.
The concentration of active IgG molecules in
affinity purified rabbit antisera to pimonidazole adducts and the unpurified
rabbit antisera to Hypoxyprobe-F6 (CCI-103F) adducts is not known.
Q: Does pimonidazole binding detect chronic and acute hypoxia?
A: Two categories of hypoxia occur in solid
tissues � diffusion-limited chronic hypoxia and perfusion-limited acute hypoxia. Chronic
hypoxia arises at the distal end of oxygen gradients created by oxygen
consumption in cells close to blood vessels compounded, in the case of tumors,
by deficiencies in local oxygen supply arising from longitudinal gradients of
pO2 in vascular trees (see Dewhirst et al., Temporal changes in pO2 of R3230AC
tumors in Fischer-344 rats, Int J Radiat Oncol Biol Phys 42: 723-726, 1998).
The presence of chronic hypoxia implies that cells in tissues consume oxygen at
a rate that is independent of oxygen supply thereby driving pO2 to very low
levels in microregions distal to blood vessels. That is, most cells possess
characteristics of a regulating� cellular phenotype that is preprogrammed to
adapt to low pO2 by smoothly transitioning to glycolytic based energy
production (see Hochachka, Patterns of O2-dependence of metabolism, Adv Exp Med
Biol 222: 143-151, 1988).
In contrast to chronic hypoxia with static,
metabolically controlled pO2 gradients, acute hypoxia is associated with
fluctuating pO2 that results from blood flow instabilities which, in the case
of tumors, is created by transient vascular occlusion. Acutely hypoxic tumor
cells, being proliferative, might be more therapeutically relevant than
quiescent, chronically hypoxic cells. In normal tissues, fluctuating hypoxia is
associated with hypoxia-reperfusion injury. With respect to whether
pimonidazole can detect both chronic and acute hypoxia, compounds that
incorporate weakly basic substituents (pKa ≥ 8.0) are concentrated in tissues
ca 3 fold above circulating blood levels. This property of weakly basic
compounds is based on the effect of differentials in intra- and extracellular
pH on intracellular concentrations of weakly basic compounds. In particular, at
pH 7.4 weakly basic 2-nitroimidazoles are concentrated intracellularly 2-fold
compared to extracellular concentration. This concentration increase is
directly reflected in increased hypoxic cell radiosensitization and labeling
with hypoxia markers. Because cells experiencing fluctuating hypoxia are
proximal to blood vessels and at relatively high pH, weakly basic,
2-nitroimidazole hypoxic markers such as pimonidazole are concentrated in these
cells whereby episodes of acute hypoxia lead to higher levels of binding
compared to hypoxia markers lacking weakly basic moieties. In this way,
pimonidazole and its analogues are superior for detecting acute hypoxia (see
Kleiter, et al. A comparison of oral and intravenous pimonidazole in canine
tumors using intravenous CCI-103F as a control hypoxia marker. Int J Radiat
Oncol Biol Phys, 64: 592-602, 2006 for further discussion and literature
cited).
Q. How soon after pimonidazole HCl administration can tissues of interest be harvested?
A: Tissues
become anoxic during harvesting and binding of circulating pimonidazole during
tissue harvest might, in principle, give false measures of hypoxia. The answer
lies in comparing tissue exposure to pimonidazole during labeling and
harvesting periods. Exposure (pimonidazole concentration x time at 37oC) is
great during the labeling period and extremely short during the harvesting
period because pimonidazole concentration is limited to that in the tissue at
the time of harvest. A combination of low marker concentration, rapid harvest
and immediate fixation in cold medium will eliminate measurable levels of
non-specific binding.
In human tumor studies, biopsies are generally
taken 16-24 hours after pimonidazole HCl infusion. The plasma half-life of
pimonidazole in humans is ca 5 hours so that 16 to 24 hours represents 3 to 5
plasma half-lives of circulating pimonidazole. This means that 1/8 to 1/32 of
the initial concentration of pimonidazole is present at the time of harvesting.
This, combined with rapid transfer of biopsy material to cold fixative,
minimizes non-specific pimonidazole binding as shown by low background binding
in the majority of cells close to blood vessels.
In some human tumor studies, biopsies were taken
1.5 to 4 hours after pimonidazole HCl infusion and biopsies immediately fixed
in liquid nitrogen. This approach also gave low background binding in cells
near blood vessels. Although the level of circulating pimonidazole is relatively
high 1.5 to 4 hours after infusion, the exposure to pimonidazole in harvested
tissue was extremely short compared to exposure to pimonidazole during the in
vivo labeling period.
The experience with human tumors can guide
experimental studies. The plasma half-life of pimonidazole in mice is typically
0.25 hours. Under these circumstances, a harvest time of 1-2 hours combined
with rapid addition to cold fixative effectively eliminates non-specific
binding. This conclusion is particularly important for experiments involving
carbon dioxide asphyxiation where the duration of global hypoxia is poorly
defined. More rapid euthanasia techniques lend themselves to shorter times of
harvest as long as rapid tissue harvest and fixation in cold fixative is
carried out.
Q. On what basis is the pO2 threshold ≤ 10 mmHg set for pimonidazole binding?
A: It is difficult to measure Km(O2) for
2-nitroimidazole binding in solid tissue. The best experiment to date is Gross
et al.抯 comparison between oxygen microelectrode
measurements of pO2 and misonidazole binding as measured by autoradiography of
radioactively labeled misonidazole in the spheroid model of solid tissue. Grain
densities due to misonidazole binding increased steeply below 10 mm Hg (Gross
et al. Calibration of misonidazole labeling by simultaneous measurement of
oxygen tension and labeling density in multicellular spheroids, Int. J. Cancer
61: 567-573, 1995). Chou et al. found that the Km(O2) for pimonidazole binding
is similar to that for misonidazole in HeLa cells and concluded that 10 mm Hg
is also a reasonable threshold value for pimonidazole binding in solid tissue
(Chou et al. Evidence that involucrin, a marker for differentiation, is oxygen
regulated in human squamous cell carcinomas. Br. J. Cancer, 90: 728-735, 2004).
A feature of solid tissues that is absent in
sparse cell cultures is that oxygen consumption creates very steep O2 gradients
so that the distance over which different Km(O2)s are traversed is
foreshortened. For example, steep pO2 gradients are observed in liver tissue
wherein immunostaining for pimonidazole adducts goes from background to intense
staining over a few cell diameters. Consistent with the presence of steep pO2
gradients in tissues are the immunostaining patterns for oxygen regulated proteins
such as involucrin and carbonic anhydrase IX that closely resemble those for
pimonidazole binding even though the Km(O2) for oxygen regulated proteins is ca
15 mm Hg compared to 2-4 mm Hg for pimonidazole binding in vitro (see Chou et
al. Evidence that involucrin, a marker for differentiation, is oxygen regulated
in human squamous cell carcinomas. Br. J. Cancer, 90: 728-735, 2004 for further
discussion).
Q. Does in vivo
N-oxidation affect pimonidazole as a hypoxia marker?
A. The piperidine moiety in pimonidazole is
easily oxidized to its N-oxide metabolite. This, in principle, could impact the
effectiveness of pimonidazole as a hypoxia marker. (See Arteel et al. Reductive
metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension
independent of the pyridine nucleotide redox state. Eur J Biochem, 253:
743-750, 1998 for a detailed discussion of the metabolism of pimonidazole). In
vivo, pimonidazole N-oxide is formed via the action of flavin mono-oxygenases
(FMO). FMO isoform distribution varies among species producing different plasma
levels of N-oxide. Interestingly, the route of pimonidazole administration (iv
or oral) has little impact on plasma levels of N-oxide (see Kleiter et al. A
comparison of oral and intravenous pimonidazole in canine tumors using
intravenous CCI-103F as a control hypoxia marker. Int J Radiat Oncol Biol Phys,
64: 592-602, 2006 for further discussion). Strong oxidants such as
peroxynitrate formed by the reaction of superoxide anion with nitrous oxide
(NO) can also oxidize pimonidazole. However, the formation of N-oxide does not
appear to compromise the effectiveness of pimonidazole as a hypoxia marker.
First, N-oxide formation is reversible by the
reducing action of heme-iron complexes in blood and by tissue reductases such
as xanthine dehydrogenase and reduced cytochrome P-450 whereby the loss of
pimonidazole by oxidation is limited (see Walton et al. The reversible
N-oxidation of the nitroimidazole radiosensitizer Ro 03- 8799. Biochem
Pharmacol, 34: 3939-3940, 1985).
Second, it has been shown by the use of a second
hypoxia marker, that the extent of hypoxia marking by pimonidazole is
independent of pimonidazole plasma concentrations at the concentration
recommended for hypoxia marking.
Third, because there is no cross reactivity
between pimonidazole and its N-oxide derivative for anti-pimonidazole
antibodies, the N-oxide does not interfere with the detection of pimonidazole
adducts in hypoxic tissues (see Kleiter et al. A comparison of oral and
intravenous pimonidazole in canine tumors using intravenous CCI-103F as a
control hypoxia marker. Int J Radiat Oncol Biol Phys, 64: 592-602, 2006 for
further discussion).
關于缺氧探針使用的更多(duō)問題歡迎咨詢,聯系電(diàn)話:021-54736159,技(jì)術(shù)QQ:498244650。
品牌故事(shì)(Brand Story):
Hypoxyprobe, Inc.(缺氧探針公司)是一(yī)家緻力于為(wèi)氧代謝紊亂引起的惡性和正常組織疾病提供診斷和治療工(gōng)具的小(xiǎo)公司。位于美國(guó)馬塞諸薩州伯靈頓(121 Middlesex Turnpike, Burlington,
Massachusetts, USA 01803),屬于美國(guó)國(guó)際天然藥物(wù)公司(Natural Pharmacia International, Inc.,NPI)缺氧探針部門(mén)的衍生(shēng)公司。
Hypoxyprobe, Inc.(缺氧探針公司)的成立起源于James
Raleigh教授(任職于查珀爾希爾(Chapel Hill)北(běi)卡羅萊納大學放(fàng)射腫瘤學部門(mén))和NPI(當時位于北(běi)卡羅萊納Research Triangle Park)之間的合作。起初的合作著(zhe)重于腫瘤缺氧的臨床研究。NPI大規模生(shēng)産缺氧探針Pimonidazole HCl,而James
Raleigh教授提供專利保護的抗體,特異性檢測人腫瘤上(shàng)結合Pimonidazole的缺氧細胞。
總體來說,使用Pimonidazole HCl作為(wèi)缺氧标記物(wù),用在狗、大小(xiǎo)鼠上(shàng)的臨床前研究越來越受科研圈的關注。2002年(nián)NPI授權五年(nián)獨家專利給Chemicon公司銷售缺氧探針試劑用于動物(wù)研究。2006年(nián)預測到(dào)市(shì)場對缺氧探針試劑的擴張需求,NPI終止chemicon授權并成立Hypoxyprobe, Inc.(缺氧探針公司)以開(kāi)發更優秀且更寬廣的試劑用于動物(wù)和臨床研究。
如今的Hypoxyprobe,
Inc.(缺氧探針公司)專注于研發非侵略性的缺氧标志(zhì)物(wù)(Hypoxia maker)和缺氧依賴性的細胞因子,所有産品皆基于Pimonidazole核心結構來開(kāi)發。